CFI
Cool Flames Investigation (CFI)

Cool flames are a very unique chemical phenomenon that can occur when many hydrocarbons and related fuels are heated in the presence of air. Cool flames are especially unique in that their chemical reaction rate first increases and then decreases with increasing temperature; this is somewhat counterintuitive and means that these flames get weaker (their heat release decreases), rather than stronger, as temperature increases over a range of temperatures, typically -700-850K (dependent on the nature of the fuel and local conditions, i.e., pressure). As the temperature increases further, however, these relatively ‘weak’ flames eventually transition to hot ignition! In effect, cool flames are a precursor to hot ignition.
Science Background
In recent ISS experiments (FLEX), a cool flame mode of burning was observed to develop after radiative extinction of a burning droplet. This result was not predicted by computational models (based on high temperature chemistry) nor expected based on prior experimental work. This unique burning behavior highlights the need to better understand both low and intermediate temperature fuel chemistry and its affect on droplet combustion, having implications for spray combustion and fire safety. This unexpected observation has attracted international interest from researchers in academia, industry, and government laboratories.
Science Application
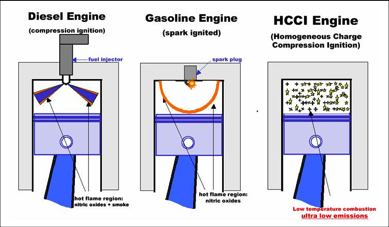
The Cool Flame Investigation (CFI) offers a unique opportunity to study the combustion of large alkane fuel droplets that exhibit hot ignition followed by radiative extinction and continued burning by low‐temperature combustion (cool flame chemistry) in the presence of heat loss. A basic understanding of cool flames is important for understanding ignition phenomena of practical fuels and fuel additives. Cool flames play a key role in the development and selection of new fuels and the design of advanced engines such as clean, high-efficiency Homogeneous Charge Compression Ignition (HCCI) engines.
The research will also help combustion chemists to develop new computational models to compute combustion performance in for many practical applications on earth, including an understanding of how cool flame reactivity determines octane and cetane number for fuels in spark ignition and diesel engines.
Understanding low temperature hydrocarbon chemistry is also very important for fire safety concerns in space.
For more background on cool flames, see “Cool Flames – Perspectives on their Importance in Combustion Systems”.
Science Objectives
1. Further understanding of the combustion characteristics of normal alkanes, particularly in the low temperature region by conducting droplet combustion experiments in low gravity with fuels that supports cool ame burning and extinction.
2. Investigate the low temperature burning behavior of droplets consisting of pure fuels and bio-fuel constituents (and mixtures of them), as well as surrogate reference fuels to determine the relationship between the cool flame burning characteristics in microgravity droplet combustion and the octane/cetane behavior of the fuel.
3. Explore the low temperature chemistry of alkanes further by mixing additives to the fuel that disrupt the low temperature chemical pathways.
For more information on the CFI Science Background and Requirements, see the CFI Science Requirements Document (SRD).
Related Documents
 |
 |
Development Approach
Milestones, pics of MDCA/ISS, etc. (overview chart)
Publications
Related Publications
- Nayagam V, Dietrich DL, Williams FA. Partial-burning regime for quasi-steady droplet combustion supported by cool flames. AIAA Journal. 2016 January 11; epub5 pp. DOI: 10.2514/1.J054437. DOI: 10.2514/1.J054437
Project Management
CFI Project Team
Science Defintion Team
Prof. Forman Williams, University of California, San Diego
Prof. Fred Dryer , Princeton University
Prof. Tanvir Farouk, University of South Carolina
Dr. Vedha Nayagam, Case Western University
Dr. Dan Dietrich, NASA John H. Glenn Research Center
Engineering Team (ZIN Technologies, Inc).
Marty O’Toole, Engineering Manager
Jess Robbins, Mechanical Engineering Lead
Joe Samrani, Electrical Engineering Lead
Steve Lawn, Diagnostic Engineering Lead
Jim Birchenough, FCF Engineering Lead
Glenn Research Center Project Staff
Andrew Suttles, Project Manager
Dan Dietrich, Project Scientist
Vedha Nayagam, Staff Scientist