FLEX
Flame Extinguishment Experiment (FLEX)

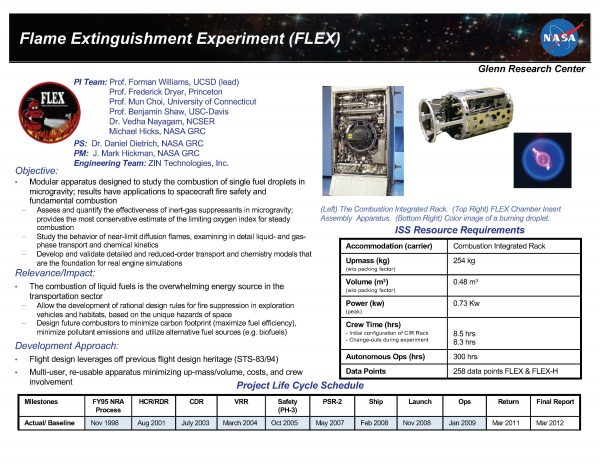
The Flame Extinguishment Experiment (FLEX) experiment was designed to assess and quantify the effectiveness of inert-gas suppressants in microgravity and obtain the most conservative estimate of the limiting oxygen index for steady combustion. FLEX is studying the behavior of near-limit diffusion flames examining in detail liquid- and gas-phase transport and chemical kinetics, and developed and is validating detailed and reduced-order transport and chemistry models that are the foundation for real engine simulations.
Flame Extinguishment Experiment (FLEX) will map the flammability boundaries for liquid fuel combustion in reduced gravity to quantify the suppressant efficacy of various gaseous suppressants over the range of candidate atmospheric pressures and oxygen concentrations. This investigation will develop predictive theoretical and numerical codes and chemical kinetic schemes to model flammability boundaries as a function effective gravitational acceleration on the unique ambient conditions encountered in space exploration applications.The development and validation of these models will require detailed spatially and temporally resolved measurements of droplet burning rate, flame extinction, flame radiation, soot concentration, soot temperature, etc. This investigation will also develop improved and validated reduced (simplified) theoretical and numerical sub-models of important physical processes (chemical kinetics, radiation, soot formation/destruction) that can be used in simulations of large scale, realistic fires.
The independent experiment variables analyzed by MDCA-FLEX are:
Oxygen Mole Fraction: The ambient oxygen mole fraction in a typical space environment can vary from high concentrations in EVA pre-breathing environments down to that typically found in air. At high oxygen concentrations, however, the chemical times are small enough (relative to the characteristic flow times) such that the droplets will burn to completion rather than exhibiting flame extinction. Therefore, it is also necessary to study low oxygen concentrations, down to the Limiting Oxygen Index (LOI). It is also important to determine the LOI in order to verify the chemical mechanisms. The oxygen mole fractions in the present study will vary from 0.10 to 0.50. The chamber must also be large enough such that there is no significant decrease in ambient oxygen mole fraction during an experiment (i.e. the droplet burns in an essentially constant ambient).
Diluent (non-suppressant): The ambient mixture will consist of oxygen mixed with a suppressant and the balance of an inert diluent gas. The diluent gas for these studies will be primarily nitrogen since that is the typical diluent on Earth and expected in space. There will be a small number of tests with a helium diluent gas. The reason is primarily for baseline comparisons with the Droplet Combustion Experiment, which used helium as the diluent gas and also to vary the physical properties of the diluent gas.
Suppressant Type: The tests will examine candidate gaseous suppressants that have widely varying physical, chemical and radiative properties. This will enable model and sub-model development and validation over a wide range of ambient conditions to improve the predictive capabilities of the models. The suppressants are carbon dioxide and helium. The expected concentrations range from 0.00 mole fraction to the limit where no flame can exist (0.70 expected for the least active suppressant).
Pressure: Ambient pressure does not significantly influence the droplet burning rate, but does influence chemistry at sufficiently low values. In addition, one strategy for extinguishing a fire is to isolate the habitat where it exists and vent the cabin to space. It is therefore beneficial to have verified suppressant data at low pressures. The pressure for the tests will range from 0.5 to 1.0 atm (standard atmosphere). As with the oxygen mole fraction, it is important that the test chamber be sufficiently large so that the pressure is essentially constant during an experiment (i.e. the droplet burns in a constant pressure ambient).
Fuel Type: The advantage of the droplet geometry is that the fuel is relatively simple and better characterized than typical fuels in fire safety studies (e.g., PMMA or paper). This is also a disadvantage since it does not represent a practical fuel. The study will use two typical hydrocarbon fuels, an alcohol, methanol (CH3OH ) and an alkane, heptane (C7H16 ). There is a relatively large experience base with these fuels. Methanol has a fuel-bound oxygen atom, and burns with a very dim blue flame (not much soot production) with a small standoff distance, so it has widely different radiative characteristics than heptane. Therefore, studying these two fuels gives a wide range of fire scenarios to verify model and sub-model performance over.
Droplet Diameter: The droplet is ignited in the flammable region and then burns in a constant oxygen mole fraction ambient until flame extinction. Under the assumption of quasisteady burning, the initial droplet size should not significantly influence the determination of the extinction droplet size. Transient influences, however, will be present, so some variation in initial droplet diameter is necessary to determine the deviation from quasi-steady behavior. The initial droplet size in the proposed study will vary between 2 and 5 mm.
Related Documents
Publications
| Publications & Presentations | ||
| FLEX and FLEX-2 Publications |
Contact Information
PI Team: Prof. Forman Williams, UCSD (lead)
Prof. Frederick Dryer, Princeton
Prof. Mun Choi, University of Connecticut
Prof. Benjamin Shaw, USC-Davis
Dr. Vedha Nayagam, NCSER
Michael Hicks, NASA GRC
PS: Dr. Daniel Dietrich, NASA GRC
PM: J. Mark Hickman, NASA GRC
Engineering Team: ZIN Technologies, Inc.