Gelled Liquid Hydrogen: A White Paper
Background
Gelled rocket propellants have been considered for many different applications.1-14 While operational usage has not yet come to fruition, there are many technology programs that are underway to eliminate the unknowns with gelled propellants and the propulsion systems that will use them. Numerous studies have shown the potential benefits of gelled fuels and oxidizers. Technology programs to prove the combustion performance of gelled propellants have been conducted most recently by the U.S. Army Missile Command, with their industry and university partners, for tactical missile applications. The NASA Lewis Research Center and its partners have investigated O2 /H2 /Al and O2 /RP-1 /Al for NASA missions and conducted experimental programs to validate elements of the combustion and fuel technology. Gelled and metallized gelled hydrogen and RP-1 have been emphasized because hydrogen and RP-1 are typical propellants for NASA launch vehicles and upper stages. Derivatives of these propellants are therefore preferred to minimize the incremental risk for a newly introduced propulsion concept. Gelled hydrogen technology is emphasized in this paper. Its likely applications would be for rocket powered launch vehicles and upper stages, rocket based combined cycle airbreathing vehicles, and combination (rocket and airbreathing) propulsion options.8-17
Why Gelled Hydrogen?
The benefits of gelled hydrogen have been known for many years and experimentally proven in the past.1-5 There are five major benefits: safety increases, boiloff reductions, density increases with the attendant area and volume related mass reductions for related subsystems (thermal protection system, structure, insulation, etc.), slosh reductions, and specific impulse (Isp) increases (in some cases).
Safety can be significantly increased with gelled fuels. A higher viscosity reduces the spill radius of the gelled hydrogen and limits the potential damage and hazard from a fuel spill. Another important advantage is the potential for leak reduction or elimination. The leak paths from the feed systems would be minimized and the possible explosion potential would be reduced.
Boiloff reduction is another feature of gelled hydrogen. The boiloff reductions are up to a factor of 2 to 3 over ungelled liquid hydrogen.8, 13, 14 This feature will assist in long term storage of hydrogen for upper stages that must sustain on-orbit storage or long coast times. Also, lunar flight and interplanetary missions with large hydrogen fuel loads will derive a benefit.
Significant density increases are possible with gelled hydrogen. A 10% density increase is possible with 10% added ethane or methane. These gellants are introduced into the hydrogen as frozen particles that form a gel structure in the hydrogen.13, 14 Appendix A provides some additional analyses of gelled hydrogen density and performance and some additional discussion of its benefits.
Specific analyses of the performance gains for various missions are dependent on the vehicle and mission design. Systems analyses performed for higher density hydrogen vehicles have shown that the reductions of the gross lift off weight (GLOW) for increased density hydrogen are very significant. In cases where another high density hydrogen, slush hydrogen was used, the density increased by 16%, the GLOW was reduced by 10.2%, or 102,000 lbm.18, 19 For airbreathing vehicles, such as the National Aerospace Plane (NASP), the estimated reduction in GLOW for slush hydrogen was from 20 to 50%. Thus, a gelled hydrogen with a 10% density increase may deliver a significant fraction of these GLOW reductions and other subsystem mass savings.20, 21
Gelled Hydrogen Density and Specific Impulse with Methane Gellants
The Isp of a gelled hydrogen powered vehicle may also increase over a liquid hydrogen powered vehicle, in some cases. Figure 1 shows the Isp variations for gelled hydrogen over an methane (CH4) percentage range of 0% to 70%. This range was selected to cover the typical values of added gellant percentages investigated in past experimental work. Also, these gellant percentages may offer attractive density increases for future vehicles. Table I provides the mixture ratios for the different methane loadings. Oxygen is the oxidizer, the expansion ratio is 40:1 and the chamber pressure is 2250 psia. A 94% Isp efficiency is used to compute the delivered Isp. The maximum Isp occurs at a 5% CH4 loading and this performance level is 4 seconds higher than ungelled O2/H2.
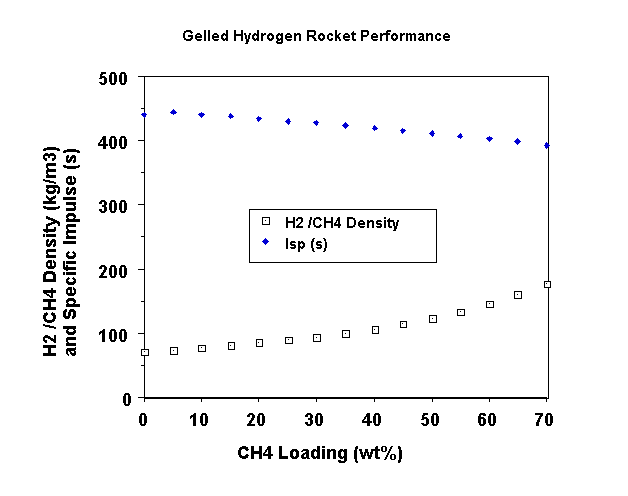
| Table I Gelled H2/CH4 Mixture Ratios and Densities |
||
| CH4 Loading (wt%) | Mixture Ratio | Density (kg/m3) |
| 0.0 | 6.0 | 70.00 |
| 5.0 | 4.2 | 73.17 |
| 10.0 | 4.2 | 76.63 |
| 15.0 | 4.2 | 80.44 |
| 20.0 | 4.3 | 84.65 |
| 25.0 | 4.3 | 89.33 |
| 30.0 | 4.3 | 94.55 |
| 35.0 | 4.2 | 100.41 |
| 40.0 | 4.3 | 107.06 |
| 45.0 | 4.2 | 114.65 |
| 50.0 | 4.2 | 123.39 |
| 55.0 | 4.1 | 133.58 |
| 60.0 | 4.1 | 145.60 |
| 65.0 | 4.0 | 160.00 |
| 70.0 | 4.0 | 177.56 |
Other Benefits of Gelled Hydrogen
For airbreathing propulsion, the largest volume of the vehicle is the hydrogen tank. Therefore, the volume reductions enabled by gelled hydrogen may be significant and this effect cascades into other subsystems for significant further mass and volume reductions. The subsystems that are affected are the aerodynamic thermal protection systems, cryogenic insulation, structural masses, and all of the other subsystems influenced by the hydrogen fuel tankage. A higher viscosity for the gel will also reduce the slosh modes in a propellant tank. Slosh baffle size and mass reductions are therefore possible by using gelled propellants. These masses can be very significant for a launch vehicle application.
Another option with gelled propellants is adding metal particles. Metallized gelled propellants may have modestly higher specific impulses (Isp increases of 5 to 6 lbf-s/lbm for O2 /H2 /Al system, 60 wt % Al in the H2 /Al fuel) compared to nonmetallized hydrogen fuels. For proposed NASA Mars evolution and expedition missions, it has been estimated that metallized gelled O2 /H2 /Al propellants can result in a 20 to 33% improvement in surface payload delivery capability.11 More importantly for O2 /RP-1 /Al and NTO /MMH /Al propellants, adding metal can deliver considerably higher propellant density, depending on the application. Hence, both the tankage mass as well as the overall propulsion system dry mass can be substantially reduced. The propellant density increases and their attendant Isp changes with the aluminum additives allow a payload increase of 14 to 35 percent by replacing the Space Shuttle Solid Rocket Booster with a Liquid Rocket Booster using O2 /RP-1 /Al and NTO /MMH /Al, respectively. 12
References
| Title | Author(s) | Source(s) | Topic | Date |
|---|---|---|---|---|
| Gelling of Liquid Hydrogen | McKinney, C.D., and Tarpley, W. | Technidyne, Inc., Contract Number NAS3-4186, NASA CR-54967, RR 66-49 | Gelled Liquid Hydrogen | June 1, 1966 |
| A Study of Hydrogen Slush and/or Hydrogen Gel Utilization - Vol. 1: Saturn S-4C Manned Mars Flyby Vehicle Application Study | Keller, C.W. | NASA CR-98936, NAS8-20342, Lockheed Missiles and Space Co., K-11-67-1K- Vol. 1 | Gelled Liquid Hydrogen | October 31, 1968 |
| A Study of Hydrogen Slush and/or Hydrogen Gel Utilization -Vol. II: Test Program 41.5-inch Diameter Tank | Keller, C.W. | NASA CR-98935, NAS8-20342, Lockheed Missiles and Space Co., K-11-67-1K-Vol. 2 | Gelled Liquid Hydrogen | October 31, 1968 |
| Carbon Compounds /Liquid Hydrogen Fuels", | E. Van der Wall | Aerojet Liquid Rocket Company Final Report, Technical Report FR02-W396, Contract SNP-1 | Gelled Liquid Hydrogen | October 1970 |
| Characteristics of a Gelled Liquid Hydrogen Polyphenylene Oxide (PPO) Foam Open-Cell Insulation System | General Dynamics, Report Number GDCA 632-3-169, Contract NAS8-27203 | Gelled Liquid Hydrogen | February 15, 1973 | |
| Advanced Gellant Materials for Metallized Cryogenic Propellants | Wong, W. | TRW Final Report, NAS3-25793 | Gelled Liquid Hydrogen | September 1993 |
| Cryogenic Gellant and Fuel Formulation for Metallized Gelled Propellants: Hydrocarbons and Hydrogen with Aluminum | Wong, W., Starkovich, J., Adams, S., and Palaszewski, B. | AIAA 94-3175, presented at the 30th AIAA/ASME/SAE/ASEE Joint Propulsion Conference | Gelled Liquid Hydrogen | June 1994 |
| Launch Vehicle and Upper Stage Liquid Propulsion at the Astronautics Laboratory (AFSC) - A History Summary | R. Wiswell and M. Huggins | AIAA 90-1839 | Gelled Liquid Hydrogen | |
| Metallized Gelled Propellant Experiences and Lessons Learned: Oxygen /RP-1 /Aluminum Rocket Engine Testing | Palaszewski, B. | in CPIA Publication 627, 1995 Gel Propulsion Technology Symposium, Huntsville, AL | Gelled Liquid Hydrogen | September 1995 |
| Metallized Gelled Propellants: Oxygen /RP-1 /Aluminum Rocket Combustion Experiments | Palaszewski, B. and Zakany, J. | AIAA 95-2435, presented at the 31st AIAA/ASME/SAE Joint Propulsion Conference, San Diego, CA | Gelled Liquid Hydrogen | July 1995 |
| Metallized Propellants for the Human Exploration of Mars | B. A. Palaszewski, " | Case for Mars IV Conference, Boulder, CO | Gelled Liquid Hydrogen | June 4-8, 1990 |
| Launch Vehicle Propulsion Using Metallized Propellants," , June 24-27, 1991, . | Palaszewski, B. and Powell, R. | NASA-Lewis Research Center, AIAA 91-2050, presented at the 27th AIAA/ASME/SAE Joint Propulsion Conference, Sacramento, CA, also in AIAA Journal of Propulsion and Power, Vol. 10, No. 6, pp. 828-833 | Gelled Liquid Hydrogen | Nov.-Dec. 1994 |
| Propulsion Systems Hazards Evaluation and Liquid/Gel Propulsion Component Development Program | G. Giola, W. Chew, D. Ryder | Volume IV - Executive Summary, TRW, Inc., Final Report, Contract Number DAAH-01086-C-0114, Technical Report CR-RD-PR-90-1 | Gelled Liquid Hydrogen | December 1989 |
| Preparative and Mechanistic Studies on Unsymmetrical Dimethyl Hydrazine-Red Fuming Nitric Acid Liquid Propellant Gels | N. Munjal, B. Gupta, and M. Varma | in Propellants, Explosives, and Pyrotechnics, 10, 4, 111 | Gelled Liquid Hydrogen | 1985 |
| A User's Primer for Comparative Assessments of All-Rocket and Rocket based Combined Cycle Propulsion Systems for Advanced Earth-to-Orbit Space Transport Applications | Escher, W., Hyde, E, and Anderson, D. | AIAA 95-2474, presented at the 31st AIAA/ASME/SAE Joint Propulsion Conference, San Diego, CA | Gelled Liquid Hydrogen | July 1995 |
| Motive Power for Next Generation Space Transports: Combined Airbreathing and Rocket Propulsion | Escher, W. | AIAA 95-6076, AIAA 6th International Aerospace Planes and Hypersonics Technologies Conference, Chattanooga, TN | Gelled Liquid Hydrogen | April 1995 |
| The Strutjet Engine: The Overlooked Option for Space Launch | Siebenhaar, A., and Bulman, M. | AIAA 95-3124, presented at the 31st AIAA/ASME/SAE Joint Propulsion Conference, San Diego, CA | Gelled Liquid Hydrogen | July 1995 |
| Cryogenic Propellant Densification Study | Elwart, R, and Dergance, R. | Martin Marietta Corp., NASA CR-159483, MCR-78-586 | Gelled Liquid Hydrogen | November 1978 |
| Advanced Technologies for Rocket Single Stage to Orbit Vehicles | Wilhite, A., et al. | AIAA Journal of Spacecraft and Rockets, Volume 28, Number 6, pp. 646-651. | Gelled Liquid Hydrogen | November-December 1991 |
| Technology Issues Associated with Using Densified Hydrogen for Space Vehicles | Hardy, T., and Whalen, M. | AIAA 92-3079, AIAA 28th Joint Propulsion Conference, Nashville, TN | Gelled Liquid Hydrogen | July 1992 |
| Benefits of Slush Hydrogen for Space Missions | Friedlander, A., Zubrin, R., and Hardy, T. | NASA Technical Memorandum 104503 | Gelled Liquid Hydrogen | October 1991 |
| Sol-Gel Science | C. J. Brinker and G. W. Scherer | Academic Press | Gelled Liquid Hydrogen | 1990 |
| Rheology of Disperse Systems | C. C. Mills | Pergamon Press, New York | Gelled Liquid Hydrogen | 1959 |
| Measurement of the Viscosity of Para-Hydrogen | D. E. Diller | J. of Chem. Phys., 42 (6), 2089 | Gelled Liquid Hydrogen | 1965 |
| The Viscosity of Liquid Hydrogen | H. E. Johns | Can. J. Res. 17A, 221 | Gelled Liquid Hydrogen | 1939 |
| Viscosity Measurement in Liquefied Gases | A. Van Itterbeek, H. Zink, and O. Van Paemel | Cryogenics, 2, 210 | Gelled Liquid Hydrogen | 1962 |
| N. S. Rudenko and V. G. Konareva | Zh. Fiz. Khim., 37, 2761 | Gelled Liquid Hydrogen | 1963 |
