The Process of Combustion
To move any airplane through the air, we must use a propulsion system to generate thrust. Different types of aircraft use different types of propulsion devices, but all aircraft rely on some type of engine to generate the power needed to run the propulsion system. The internal combustion, or piston engine, used by the Wright brothers and the modern jet and rocket engine all depend on the burning of fuel to produce power. Burning a fuel is called combustion, a chemical process that we study in middle or high school.
Because combustion is so important for aircraft and rocket propulsion, we will review the fundamentals. Combustion is a chemical process in which a substance reacts rapidly with oxygen and gives off heat. The reacting substance is called the fuel, and the source of oxygen is called the oxidizer. The fuel can be a solid, liquid, or gas. For airplane propulsion the fuel is usually a liquid hydrocarbon, like gasoline. The oxidizer, likewise, can be a solid, liquid, or gas. For airplane propulsion the oxidizer is air, a gas. Since the oxidizer is a gas and the fuel is a liquid, we have to vaporize the fuel for combustion to occur in an airplane engine. The vaporization and mixing of the fuel and air occur in the carburetor of the engine, the combustion itself occurs in the combustion chamber.
During combustion, new chemical substances are created from the fuel and the oxidizer. These substances are called exhaust. Most of the exhaust comes from chemical combinations of the fuel and oxygen. When a hydrogen-carbon-based fuel (like gasoline) burns, the exhaust includes water (hydrogen + oxygen) and carbon dioxide (carbon + oxygen). But the exhaust can also contain chemical combinations of other elements present during combustion. If gasoline is burned in air, which contains 21% oxygen and 78% nitrogen, the exhaust can also include nitrous oxides (NOX, nitrogen + oxygen). The exhaust is usually in the form of a gas because of the high temperature from the heat released. But some liquid and even solid exhaust can be formed. (Example: Soot is a form of solid exhaust that occurs in some combustion processes.)
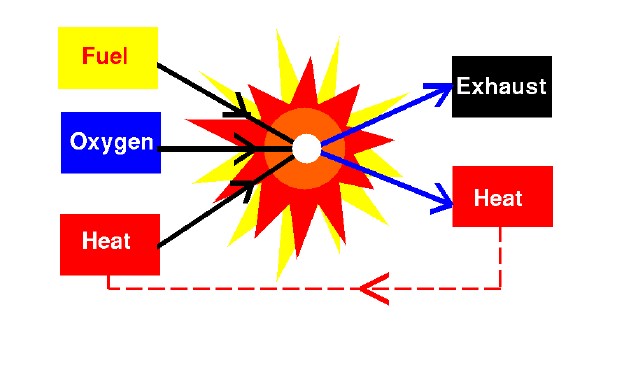
During the combustion process, as the fuel and oxidizer are turned into exhaust products, heat is generated. Interestingly, some source of heat is also necessary to start combustion. (Gasoline and air are both present in your automobile fuel tank; but combustion does not occur because there is no source of heat.) Since heat is both required to start combustion and is itself a product of combustion, we can see why combustion takes place very rapidly. Also, once combustion gets started, we don’t have to continually provide a heat source. The heat of combustion will keep things going. (Example: We don’t have to keep lighting a campfire once it is burning.)
To summarize, for combustion to occur three things must be present: a fuel to be burned, a source of oxygen, and a source of heat. As a result of combustion, exhausts are created and heat is released. You can control or stop the combustion process by controlling the amount of the fuel available, the amount of oxygen available, or the source of heat.