Air Temperature
What is Temperature?
An important property of any gas (including air) is temperature. We have some experience with temperature that we don’t have with properties like viscosity and compressibility. We’ve heard meteorologists give the daily value of the temperature of the atmosphere (15 degrees Celsius, for example). We know that a hot object has a high temperature, and a cold object has a low temperature. And we know that the temperature of an object can change if we heat the object or cool it.
Scientists, however, must be more precise than simply describing an object as “hot” or “cold.” An entire branch of physics, called thermodynamics, is devoted to studying the temperature of objects and the flow of heat between objects of different temperatures. We are including some fundamentals of thermodynamics at the Wright brothers web site to help you better understand the fundamentals of engines.
There are two ways to look at temperature: (1) the small scale action of individual air molecules or (2) the large scale action of a large number of molecules. The small scale action is described by the kinetic theory of gases and we have no evidence that the Wright brothers were familiar with this theory when they designed the aircraft engine.
The most important result from the small scale view of temperature is that the temperature of a gas is related to the average kinetic energy of the moving gas molecules. Kinetic energy is mass times the velocity squared. So the higher the temperature of a gas, the higher the velocity of the molecules of the gas; heating a gas causes the molecules to move at a higher velocity. The pressure of a gas depends on the momentum (mass times velocity) of the molecules, so temperature and pressure are related through an equation of state.
Principles of Thermodynamics
Considering the large scale effects, the temperature of a gas is something that we can determine qualitatively with our senses. We can sense that one gas is hotter than another gas and therefore has a higher temperature. But to determine the temperature quantitatively, to assign a number, we must use some principles from thermodynamics:
- The first principle is the observation that the temperature of an object can affect some properties of the object, such as the length of a solid, or the gas pressure in a closed vessel, or the electrical resistance of a wire. (Changing the temperature changes the length of a solid bar.)
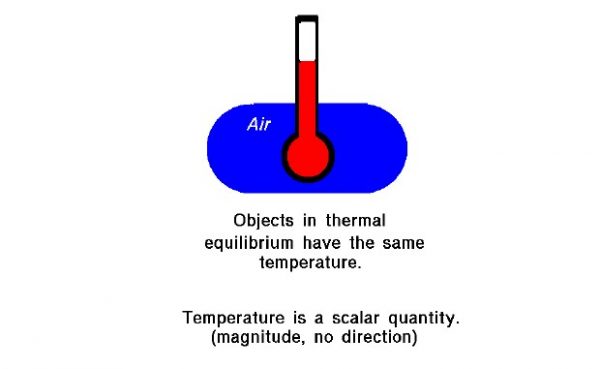
- The second principle is the definition of thermodynamic equilibrium between two objects. Two objects are in thermodynamic equilibrium when they have the same temperature.
- And the final principle is the observation that if two objects of different temperatures are brought into contact with one another, they will eventually establish a thermodynamic equilibrium. (The word “eventually” is important. Insulating materials reach equilibrium after a very long time, while conducting materials reach equilibrium very quickly.)
With these three thermodynamic principles, we can construct a device for measuring temperature, a thermometer, which assigns a number to the temperature of an object. When the thermometer is brought into contact with another object, it quickly establishes a thermodynamic equilibrium. As the temperature is changed, the thermodynamic effect is changed (the length of the mercury in the tube changes). By measuring the thermodynamic effect on the properties of the thermometer at some reference conditions (like the boiling point and freezing point of water) we can establish a scale for assigning temperature values.
Celsius and Fahrenheit Scales
The number assigned to the temperature depends on what we pick for the reference condition. So several different temperature scales have arisen. The Celsius scale, designated with a C, uses the freezing point of pure water as the zero point and the boiling point as 100 degrees with a linear scale in between these extremes. The Fahrenheit scale, designated with an F, is a lot more confusing. It originally used the freezing point of sea water as the zero point and the freezing point of pure water as 30 degrees, which made the temperature of a healthy person equal to 96 degrees. On this scale, the boiling point of pure water was 212 degrees. So he adjusted the scale to make the boiling point of pure water 212 and the freezing point of pure water 32, which gave 180 degrees between the two reference points. 180 degrees was chosen (as it is for a circle) because it is evenly divisible by 2, 3, 4, 5, and 6. On the new temperature scale, the heat of a healthy person is 98.6 degrees F. Because there are 100 degrees C and 180 degrees F between the same reference conditions:
1 degree C = 1 degree F * 100 / 180 = 1 degree F * 5 / 9
Since the scales start at different zero points, we can convert from the temperature on the Fahrenheit scale (TF) to the temperature on the Celsius scale (TC) by using this equation:
TF = 32 + (9 / 5) * TC
Absolute Temperature
Of course, you can have temperatures below the freezing point of water and these are assigned negative numbers. When scientists began to study the coldest possible temperature, they determined an absolute zero at which molecular kinetic energy is a minimum (but not strictly zero!). They found this value to be at -273.16 degrees C. Using this point as the new zero point we can define another temperature scale called the absolute temperature. If we keep the the size of a single degree to be the same as the Celsius scale, we get a temperature scale which has been named after Lord Kelvin and designated with a K. Then:
K = C + 273.16
There is a similar absolute temperature corresponding to the Fahrenheit degree. It is named after the scientist Rankine and designated with an R.
R = F + 459.69
Absolute temperatures are used in the equation of state and the derivation of the state variables enthalpy and entropy, which are used to solve gas dynamics problems. Temperature, like pressure, is a scalar quantity; it has no direction associated with it. It has just a single value at every location in a gas. The value can change from location to location, but there is no direction connected to the temperature.
