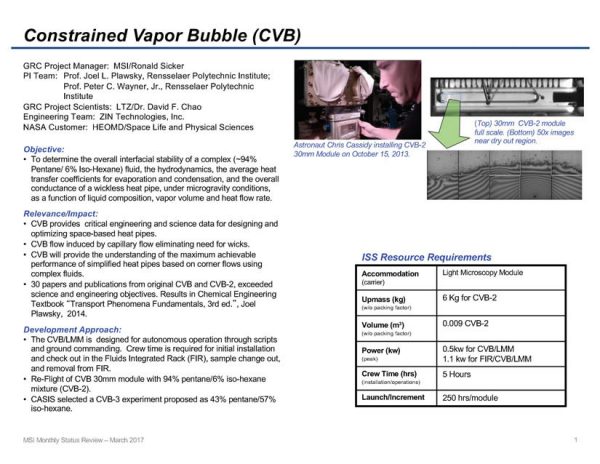
Constrained Vapor Bubble-2 (CVB-2)

CVB-2 is a continuation of the original CVB investigation, where the goal is the study of thermophysical principles underlying change-of-phase heat transfer systems. In CVB-2 the effect of replacing a pure fluid (100% Pentane used in CVB) with a mixture (94% Pentane and 6% Isohexane in CVB-2) is observed. The study of the mixture will evaluate the effect of a modified liquid/vapor interfacial shear stress on fluid flow towards the zone of evaporation. Most liquids have a surface tension that decreases with increasing temperature. This phenomenon creates a flow in the direction of increasing surface tension, called “Marangoni Flow”. The temperature driven Marangoni Flow reduces the effectiveness of a heat pipe like the CVB because it keeps liquid from returning to the hot end. The use of a liquid mixture can mitigate this effect because the change in mixture composition with temperature opposes the change in surface tension with temperature allowing for liquid to keep flowing toward the hot end of the device. This should change the details of the basic evaporation and condensation processes operating in the cell.
The crewmember determines the pressure gradient driving liquid flow through the heat-exchanger optically by measuring the shape of the vapor-liquid interface. Temperature gradients driving the heat flow are measured using a series of thermocouples drilled into the side of the CVB cell. Due to their sensitivity to gravity and to small temperature and pressure gradients, heat pipe transport systems like the CVB need to be studied under microgravity conditions to obtain some essential, fundamental information about their internal fluid recirculation systems operate.
Part of the rationale for the experiment is to understand how mixtures of fluids behave during evaporation/condensation cycles. This has applications in chemical processes like boiling, evaporation, condensation, refrigeration, distillation, and in stripping processes where a volatile toxic compound would need to be removed from say, groundwater. The operation of the CVB-2 device also provides fundamental reference data that people who work on advanced micro- and nano- structured heat exchange surfaces need to benchmark their inventions. There are also applications to space-based and terrestrial heat pipes. All high performance laptop computers now incorporate heat pipes to keep their processors cool. Most satellite systems also use heat pipes to keep critical components cool. Fluid mixtures have been reported to perform better, allowing heat pipes incorporating them to be smaller and lighter.
In general, there are many devices where overheating (e.g., computers or LED light bulbs) can seriously degrade performance and lifetime and so need perpetual cooling. Since heat pipes use capillary action instead of a mechanical pump to supply the cooling fluid (which evaporates) to the hot region, they are a sophisticated, quiet, light, reliable, and efficient method to transfer the heat from the hot region. The objective of CVB and CVB-2 is to understand the details of the evaporation/condensation/fluid-flow operation of these devices and thereby lead to better designs. Heat pipes are particularly useful in microgravity because they eliminate the need for a mechanical pump, a primary source of failure in most systems. Since the effect of gravity is absent in microgravity, the process by which a heat pipe operates is also simpler on the ISS and easier to study. However, the results of the study would also be applicable on earth. In our studies, we compare the operation of the same device in 1g and microgravity. Thereby, adding to the general usefulness of the results of the study
NASA and ZIN Technologies developed the experimental setup for flight aboard the International Space Station as a part of the Fluids Integrated Rack (FIR). The Light Microscopy Module (LMM) developed as a part of the FIR consists of a completely automated optical microscope performs a variety of experiments.
Science Objectives
Science Objectives for Everyone
Constrained Vapor Bubble-2 (CVB-2) uses a miniature heat pipe and a mixture of two fuels to investigate the physics and engineering of heat transfer systems. The investigation conducts basic research in thermodynamics, including heat transfer and the phenomena that occur at the boundary between two phases of matter. It also studies the effectiveness of heat transfer using the CVB heat pipe, a passive heat transfer system on the International Space Station.
Science Results for Everyone
Chill out! Devices such as computers need constant cooling to perform well. Small, liquid-filled pipes are one way to transfer heat without pumps or fans. Researchers tested mixtures of water and alcohol in heat pipes in space. Results revealed an ideal mixture of pentane and isohexane that transferred more heat than other fluids, and eliminated common problems caused by changes in fluid surface tension and location of bubble formation in the pipe. These insights into how heat transfers in microgravity and how mixtures of fluids behave have applications in space and a variety of industries on Earth.
Applications
Space Applications
Most liquids have a surface tension that decreases as temperature rises. This causes fluid to flow in the direction of higher surface tension, a phenomenon known as Marangoni flow. This reduces the effectiveness of a heat pipe, because liquid cannot return to the hot end. CVB-2 investigates whether a mixture of two fuels (pentane and isohexane) that can reduce Marangoni flow and improve the efficiency of the CVB system on the International Space Station. In addition, the investigation provides insight into how heat transfer functions in microgravity. Most satellites use heat pipes to keep critical parts cool, and improved understanding of fluid mixtures could enable future heat pipes that are smaller, lighter and less expensive to launch.
Earth Applications
A wide range of high-performance devices must be perpetually cooled in order to function, from supercomputers to personal laptops and LED light bulbs. Fluid mixtures circulating through heat pipes can passively dissipate heat, using less energy than fans or cooling systems. But heat pipe transport systems are sensitive to small differences in temperature, pressure, and gravity. Studying how fluid mixtures and heat pipes function in microgravity provides insight into the fundamental principles of thermodynamics. This also provides insight into how mixtures of fluids behave during boiling, condensation, evaporation, distillation, and stripping. These processes are used in a variety of industries and environmental cleanup.
Related Documents
Data is currently unavailable.
Publications
CVB-2 studied heat transfer systems, using a heat pipe. A heat pipe contains a fluid that moves within it, transferring heat without a pump. Recently, several research groups used fluids containing water and a low concentration of alcohol. This resulted in better performance of the heat pipe. The alcohol/water combinations were peculiar in that for a certain composition range, the surface tension increases with increasing temperature. This caused the liquid to move toward the hotter end of the pipe. For the first time, the team used an ideal fluid mixture of 94 percent-by-volume pentane and 6 percent-by-volume isohexane as the working fluid. Using a simple heat transfer model developed in their laboratory, they determined an internal heat transfer coefficient in the evaporator section. They showed this coefficient to be almost twice that of pure pentane under the same conditions. The Marangoni stress in the mixture was five times lower, which led to less liquid accumulation near the heater end. Images of the device showed that the bubble gets much closer to the heater end in the mixture case. This differed from the original CVB experiment, when the bubble was isolated from the heater by a thick liquid pool. The proximity of the bubble to the heater wall led to more evaporation at the heater end in the mixture case, and therefore a higher heat transfer coefficient. Experiments with heat pipes provide insight into how mixtures of fluids behave during boiling, condensation, evaporation, distillation, and stripping. A variety of industries, including environmental cleanup, use these processes.
Results Publications
- Nguyen TT, Kundan A, Wayner, Jr. PC, Plawsky JL, Chao DF, Sicker RJ. The effect of an ideal fluid mixture on the evaporator performance of a heat pipe in microgravity. International Journal of Heat and Mass Transfer. 2016 April; 95765-772. DOI: 10.1016/j.ijheatmasstransfer.2015.12.032. DOI: 10.1016/j.ijheatmasstransfer.2015.12.032
- Nguyen TT, Kundan A, Wayner, Jr. PC, Plawsky JL, Chao DF, Sicker RJ. Effects of cooling temperature on heat pipe evaporator performance using an ideal fluid mixture in microgravity. Experimental Thermal and Fluid Science. 2016 July; 75108-117. DOI: 10.1016/j.expthermflusci.2016.01.016. DOI: 10.1016/j.expthermflusci.2016.01.016
Gallery
Contact Information
Project Manager: Ronald J. Sicker, NASA GRC
Ronald.J.Sicker@nasa.gov
216-433-6498
Project Scientist: Dr. David F. Chao, NASA GRC
David.F.Chao@nasa.gov
216-433-8320
Principal Investigator: Prof. Peter C. Wayner, Jr., Rensselaer Polytechnic Institute
wayner@rpi.edu