Gas Sensors
Hydrogen Sensing Technology
In launch applications, hydrogen leaks pose significant operational safety concerns. In response, NASA’s Glenn Research Center developed microfabricated point-contact hydrogen sensors for finding the position of the leaks. One component of this program involves the fabrication of Pd-alloy hydrogen sensors on Si substrates. The hydrogen sensor includes a Schottky diode and a hydrogen sensitive resistor for detection from the ppm range to 100% hydrogen (shown below).
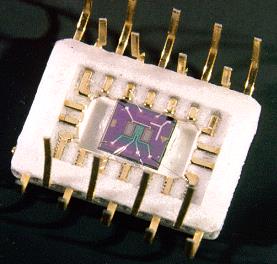
The sensor includes a temperature detector and heater for operation in a wide variety of environments. It has high sensitivity and can operate in either inert or oxygen-containing environments. The sensor’s operating range can be designed from ppm to 100%.
These sensors have been commercialized by Makel Engineering, Inc. into a complete, wide range, leak detection system. They are part of a safety system on the International Space Station, have flown or been used in multiple applications, and are presently used by Blue Origin. The basic platform structure can also be used for the detection of toxic gases such as hydrazine.
A second component of the program involves the use of silicon carbide (SiC) rather than silicon (Si) as the semiconductor in a Schottky diode structure. The use of SiC has been demonstrated for applications that require high-temperature detection of hydrogen in conditions in which Si-based technology will not function. High-temperature detection is further discussed in the Hydrocarbon Sensing Technology section.
Hydrocarbon Sensing Technology
The detection of hydrocarbons is important in the monitoring of aeronautic engine exhaust, leak detection, and fire detection. For example, aeronautic gas emissions are often at temperatures considerably above the threshold where the silicon semiconductor hydrogen sensor is operable. Furthermore, it is at these higher temperatures that catalytic effects occur that often make hydrocarbon detection possible.
For these higher temperature applications, a Schottky diode using silicon carbide (SiC) rather than Si semiconductor technology has been developed. The material properties of SiC make it suitable for operation in hostile conditions, which exceed the inherent limitations of Si-based electronic devices. In particular, the ability of SiC to operate as a semiconductor at temperatures higher than 600ºC makes it useful in high-temperature emission measuring applications, as well as for the development of high-temperature electronics.

Thus, the use of a SiC rather than Si semiconductor in a Schottky diode structure allows this platform to be used at high temperatures. The use of precious metal catalysts in the Schottky diode structure allows the sensor to respond to hydrocarbons with high sensitivity. After notable development, the sensor has shown stability at high temperature for long durations. Further, the same sensor structure with internal temperature control can operate in applications that are not at high temperatures but also require the need for sensitive hydrogen or hydrocarbon detection.
A SiC-based Schottky diode hydrocarbon and hydrogen sensor package including a temperature detector and heater has been applied in multiple applications including leak detection, emissions monitoring, and fire detection. Advancements in this area have been patented and were nominated for NASA Invention of the Year in 2009 and received honorable mention for the 2017 NASA Invention of the Year. Present work includes integrating such sensors as these with SiC-based high-temperature electronics
Nitrogen Oxide Sensing Technology
NASA’s Glenn Research Center has developed multiple methods of nitrogen oxide (NOx) detection. One approach, in a previous collaboration with The Ohio State University, is based on a solid-state electrochemical NOx sensor for breath monitoring.

The emphasis of this work was on the miniaturization of the sensor based on larger sensor technology. Due to its capability of resisting high temperatures, the sensor could also be extended to applications such as monitoring NOx from a jet engine.
Research at NASA Glenn focused on miniaturizing these sensors as part of a program with the Cleveland Clinic Foundation with funding provided by the state of Ohio Third Frontier program. Alternate approaches are based on SiC Schottky diodes or other electrochemical cell structures.
Carbon Monoxide Sensing Technology
The detection of carbon monoxide (CO) is necessary for a range of applications. There is a need for improved CO sensors for fire detection and environmental monitoring. CO detection is also of interest in aeronautic emissions applications. NASA’s Glenn Research Center has supported the development of two major types of CO sensor technology: one based on tin oxide (SnO2), and the other based on titanium dioxide (TiO2). Both have been miniaturized for smart sensor applications.
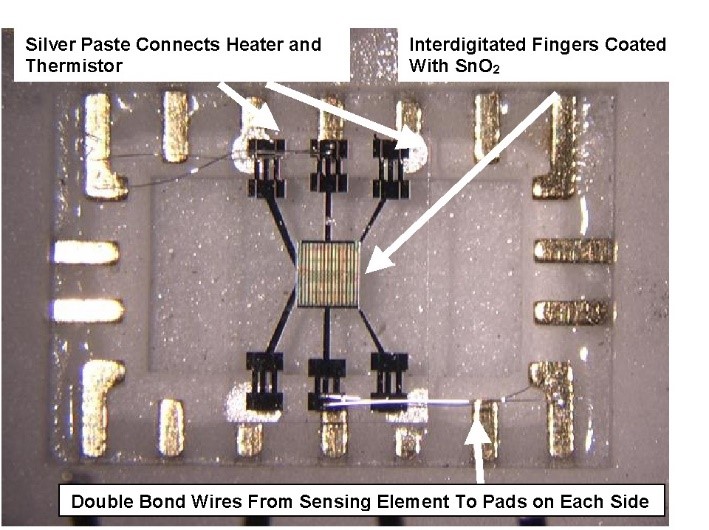
For example, one CO sensor type uses specially processed tin oxide (SnO2) as the gas sensitive material to ensure stable operation. A standard characteristic of SnO2 is that it undergoes drift at high temperatures due to annealing of the grains. However, nanocrystalline SnO2 provides greater stability and sensitivity at higher temperatures due to its small grain size and large surface area and is thus more useful as a sensor. By doping (through the addition of impurities, such as another element) the SnO2 appropriately, the sensor can be fine-tuned to become more sensitive to either CO or NOx.
Oxygen Sensing Technology
Multiple types of oxygen sensors have been developed using an electrochemical cell platform. This has ranged from sensors that operate at high temperature with zirconia electrolyte to one that runs at room temperature with NAFION electrolyte.
For example, a microfabricated O2 sensor has been developed for safety purposes in aerospace applications, but significant applications also exist in the area of aeronautic emissions control and human health applications.
Conventional oxygen sensors utilize an electrochemical cell containing ZrO2, which conducts oxygen ions at high temperatures. These standard cells are bulky and power consuming. The oxygen sensors developed contain a complete microfabricated electrochemical cell mounted onto a small chip.
The main benefits of this sensor approach includes a wider oxygen concentration measurement range, less power consumption, and cheaper production costs.
A room temperature version of this sensor has been developed using a different electrolyte. NAFION solution doped with water-retaining components are coated on the electrode area to form a conducting electrolyte layer. The sensor is operated under potentiometric mode, which means electrical potential difference was measured between these two electrodes.
Carbon Dioxide Sensing Technology
Research has been on-going to create smaller, cheaper, and more accurate CO2 sensors with a wide temperature range.
Combined with the CO sensors, these devices would have important fire detection applications as well as in aeronautic applications in emissions monitoring and human health monitoring. Similar in basic approach to the oxygen sensors, these CO2 sensors make use of a solid electrochemical cell.
In this design, the solid electrolyte used is NASICON (sodium super ionic conductor). Stable and repeatable operation of this sensor has been demonstrated for significant periods of time and improved operation of the sensor has been shown by the addition of nanomaterials. This device is microfabricated to enable the benefits of lower production and operating costs.
Nanoplasmonic sensing
Nanoplasmonic sensors exploit the phenomenon of ‘surface plasmon resonance’—coupled electron–electromagnetic wave oscillations a metal–dielectric interface under incident illumination by light. These resonant surface waves have confined/enhanced electric fields, which are highly sensitive to changes in the local dielectric environment, e.g., the binding of a biomolecule or chemical species. The resulting change in dielectric environment is transduced as a shift in the resonance feature. Surface functionalization is required to ensure sensor specificity, and we are exploring different approaches depending on the target application, including polymer-based recognition elements, templated ’lock-in-key’/artificial antibody approaches, and bio-affinity interactions (e.g., DNA/RNA hybridization).
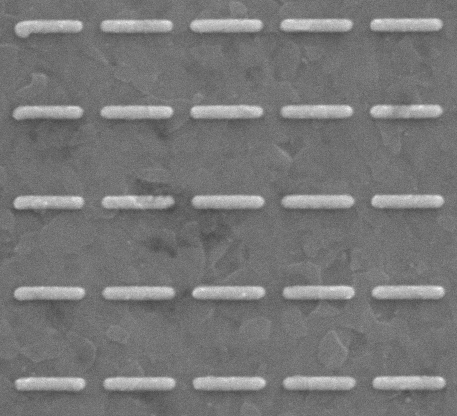
A primary goal of this work is to transition this sensing technology out of the lab, where it often relies on lab-based instrumentation (microscope, spectrometer, etc.) for signal excitation and readout, to the portable, field/space-deployable systems. We are investigating different sensor geometries, which enable integration with on-chip photonic integrated circuits, while maintaining high sensitivity and the other desirable properties of nanoplasmonic sensing.
Benefits of nanoplasmonic sensors for NASA’s mission: low-temperature operation/potential for in-situ measurements (e.g., Icy Moon/Ocean Worlds), tailorable recognition elements for a wide range of applications, real-time and label-free operation.
